Abstract
Introduction: Brexucabtagene autoleucel (brexu-cel, formerly known as KTE-X19) is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved in the United States for the treatment of adults with R/R B-ALL based on the results of the pivotal Phase 2 ZUMA-3 study. After 2 years of follow-up in ZUMA-3, brexu-cel demonstrated a high overall complete remission (CR) rate (CR + CR with incomplete hematologic recovery [CRi]) of 71%, with a median relapse-free survival (RFS; censored at subsequent stem cell transplant [SCT]) of 11.6 months and a median overall survival (OS) of 25.4 months in 55 treated adults with R/R B-ALL (Shah et al, ASCO 2022). To better assess the unmet need and the benefit of brexu-cel in adults with R/R B-ALL, the retrospective historical control study SCHOLAR-3 was conducted (Shah et al, ASH 2021). Here, we report updated outcomes in SCHOLAR-3 with longer follow-up.
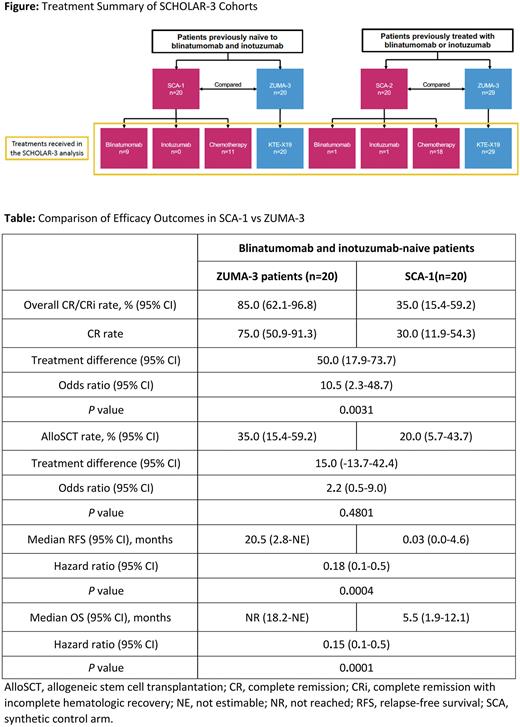
Methods: In ZUMA-3 (NCT02614066), patients (≥18 years) with R/R B-ALL received a single infusion of brexu-cel (1×10⁶ CAR T cells/kg) following leukapheresis and conditioning chemotherapy (Shah et al, Lancet 2021). Detailed SCHOLAR-3 methodology was previously reported (Shah et al, ASH 2021). Briefly, propensity scoring was used to match ZUMA-3 treated patients with adult patients with R/R B-ALL treated in historical clinical trials (synthetic control arm [SCA]) based on key baseline characteristics and prior therapies. The study consisted of 3 patient-matched historical control cohorts: (1) SCA-1: patients who were previously naive to blinatumomab and inotuzumab prior to enrollment to the historical clinical trial; (2) SCA-2: patients who were previously treated with blinatumomab or inotuzumab prior to enrollment to the historical clinical trial; and (3) SCA-combined: SCA-1 and SCA-2 combined data set (Figure). These cohorts were compared with matched ZUMA-3 patients who were previously naive to blinatumomab and inotuzumab prior to enrollment in ZUMA-3, previously treated with blinatumomab or inotuzumab prior to enrollment in ZUMA-3, and any pretreatment status, respectively (Figure). The primary endpoints were CR/CRi rate at 24 weeks for patients previously naive to blinatumomab and inotuzumab and OS for all cohorts.
Results: At data cutoff, the median follow-up time for the SCHOLAR-3 analysis was 18.6 months. Baseline characteristics were previously reported (Shah et al, ASH 2021). For patients previously naive to blinatumomab and inotuzumab in ZUMA-3 (n=20) vs SCA-1 (n=20), the CR/CRi rates at 24 weeks were 85% (95% CI, 62.1-96.8) vs 35% (95% CI, 15.4-59.2; P=.0031); the medians for RFS were 20.5 (2.8-not evaluable [NE]) vs 0.03 months (95% CI, 0.0-4.6; HR, 0.18 [95% CI, 0.1-0.5]; P=.0004); and the medians for OS were not reached (95% CI, 18.2-NE) vs 5.5 months (95% CI, 1.9-12.1; HR, 0.15 [95% CI, 0.1-0.5]; P=.0001), respectively (Table). The allogeneic stem cell transplantation rates were 35% (95% CI, 15.4-59.2) vs 20% (95% CI, 5.7-43.7; P=.4801) for ZUMA-3 and SCA-1, respectively (Table).
For patients previously treated with blinatumomab or inotuzumab in ZUMA-3 (n=29) vs SCA-2 (n=20), the median OS was 15.9 (95% CI, 3.2-26.0) vs 4.8 months (95% CI, 2.7-12.4; HR, 0.55 [95% CI, 0.3-1.1]), respectively. In ZUMA-3 (n=49) vs SCA-combined (n=40), the median OS was 25.5 (95% CI, 15.9-NE) vs 5.5 months (95% CI, 3.3-9.2; HR, 0.32 [0.2-0.6]; P=.0001), respectively. For the intention-to-treat analysis, the median OS for all matched ZUMA-3 patients (N=65) was 23.1 (95% CI, 9.9-NE) and 6.0 months (95% CI, 4.2-7.3) for all matched SCA patients (N=65; HR, 0.47 [95% CI, 0.3-0.8]; P=.0011).
Conclusions: Outcomes of patients treated in historical standard-of-care trials were poor regardless of prior therapy status (blinatumomab/inotuzumab-treated or -naive), with a median OS of less than 6 months. In contrast, matched ZUMA-3 patients achieved a median OS of >25 months, more than 4 times that of the SCA arm, highlighting a considerable benefit of KTE-X19 over standard-of-care therapies in this patient population. These results suggest that brexu-cel may improve outcomes compared to historical standard-of-care therapies and helps to address an unmet need for patients with R/R B-ALL.
Disclosures
Shah:PeproMene Bio: Other: Steering committee; Servier: Other: grants and investigator-initiated trials; Autolus: Consultancy; Century Therapeutics: Consultancy; Adaptive: Consultancy; Pharmacyclics: Consultancy; Beigene: Consultancy; Acrotech: Consultancy; Jazz: Consultancy, Other: grants and investigator-initiated trials; Precision Biosciences: Consultancy; Kite/Gilead: Consultancy, Other: grants and investigator-initiated trials; BMS/Celgene/Juno: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Amgen: Consultancy. Ghobadi:Amgen: Consultancy, Research Funding; Celgene: Consultancy; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Atara: Consultancy; Wugen Inc: Consultancy. Oluwole:Pfizer: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Curio Science: Consultancy; TG Therapeutics: Consultancy; Kite, a Gilead Company: Research Funding; ADC Therapeutics: Consultancy. Logan:Amgen: Research Funding; Amphivena: Research Funding; Astellas: Research Funding; Autolus: Research Funding; Jazz: Research Funding; Kadmon: Research Funding; Kite/Gilead: Research Funding; BMS: Consultancy; Pfizer: Consultancy; Pharmacyclics: Research Funding; Abbvie: Consultancy. Boissel:GILEAD: Honoraria; AMGEN: Honoraria; ARIAD/INCYTE: Honoraria; ASTELLAS: Honoraria; NOVARTIS: Honoraria; SERVIER: Honoraria. Cassaday:Jazz: Consultancy; Merck: Research Funding; Servier: Research Funding; Vanda: Research Funding; Pepromene Bio: Membership on an entity's Board of Directors or advisory committees; Autolus: Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Seagen: Current Employment, Current equity holder in private company, Other: Spouse employment with Seagen; stock or other ownership in Seagen.; Amgen: Consultancy, Research Funding. Leguay:Servier: Consultancy; Amgen: Consultancy. Bishop:Immatics: Research Funding; Triumvira: Research Funding; Autolus: Consultancy, Research Funding; Arcellx: Consultancy, Research Funding; WindMIL Therapeutics: Consultancy; Bluebird Bio: Consultancy; Iovance: Consultancy; CRISPR Therapeutics: Consultancy, Research Funding; Agios: Consultancy, Honoraria, Other: Travel support, Speakers Bureau; Bristol Myers Squibb: Honoraria, Other: Travel support, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: Travel support , Research Funding; Sanofi: Honoraria, Speakers Bureau; Celgene: Honoraria; Incyte: Honoraria, Other: Travel support , Speakers Bureau; Tmunity: Research Funding; Chimeric Therapeutics: Consultancy; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding, Speakers Bureau; Sana Biotechnology: Consultancy; ADC Therapeutics: Speakers Bureau; Servier: Speakers Bureau. Topp:MacroGenics: Research Funding; Regeneron: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Celgene: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Amgen: Consultancy, Research Funding. Tzachanis:Bristol Myers Squibb: Consultancy, Research Funding; Partner: Consultancy; Takeda: Consultancy; EUSA: Consultancy. O'Dwyer:BEAM Therapeutics: Consultancy. Arellano:Syndax Pharmaceuticals: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding. Lin:Celgene: Consultancy, Research Funding; Bluebird Bio: Consultancy, Research Funding; Takeda: Research Funding; Merck: Research Funding; Vineti: Consultancy; Legend: Consultancy; Novartis: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Juno: Consultancy; Sorrento: Consultancy; Janssen: Consultancy, Research Funding; Gamida Cell: Consultancy. Baer:Forma: Research Funding; Kite, a Gilead Company: Research Funding; Takeda: Research Funding; Ascentage: Research Funding; Kura Oncology: Research Funding; AbbVie: Research Funding. Schiller:FujiFilm: Research Funding; CTI: Research Funding; Daiichi-Sankyo: Research Funding; Millennium: Research Funding; Medimmune: Research Funding; Constellation: Research Funding; Cellerant: Research Funding; AVM Biopharma: Research Funding; Glycomimetics: Research Funding; Deciphera: Research Funding; Arog: Research Funding; Actuate: Research Funding; Celgene: Consultancy, Research Funding, Speakers Bureau; Ono Pharma: Honoraria; Novartis: Honoraria, Other: Speaker fees, Research Funding; AstraZeneca: Honoraria; Johnson & Johnson: Current equity holder in publicly-traded company; Amgen: Current equity holder in publicly-traded company, Honoraria; Stemline: Research Funding; Samus: Research Funding; Regimmune: Research Funding; Mateon: Research Funding; Geron: Research Funding; Genentech-Roche: Research Funding; Forma: Research Funding; Stemline: Speakers Bureau; Jazz: Consultancy; Pfizer: Research Funding; PreCOG LLC: Research Funding; Kite, a Gilead Company: Research Funding, Speakers Bureau; Deltafly: Research Funding; Gilead: Research Funding; AltruBio: Research Funding; Trovagen: Research Funding; Sellas: Research Funding; Cellectis: Research Funding; Cyclacel: Research Funding; Bristol Myers Squibb: Current equity holder in publicly-traded company, Speakers Bureau; AbbVie: Research Funding, Speakers Bureau; Janssen: Research Funding; Agios: Consultancy, Honoraria; Karyopharm: Research Funding, Speakers Bureau; Incyte: Other: speaker fees, Research Funding, Speakers Bureau; Gamida: Research Funding; Astellas: Research Funding, Speakers Bureau; Actinium: Research Funding; Onconova: Research Funding; Sangamo: Research Funding; Takeda: Research Funding; Tolero: Research Funding. Park:Amgen: Consultancy; Allogene Therapeutics: Membership on an entity's Board of Directors or advisory committees; Affyimmune Therapeutics, Inc.: Consultancy; Bristol-Myers Squibb: Consultancy; Curocell Inc.: Consultancy; Innate Pharma: Consultancy; Intellia: Consultancy; Kite, a Gilead Company: Consultancy; Kura Oncology: Consultancy; Novartis: Consultancy; Servier: Consultancy, Other: Provision of Services; AstraZeneca: Consultancy; Genentech: Research Funding; Juno: Research Funding; Autolus Therapeutics: Consultancy; Artiva Biotherapeutics, Inc.: Membership on an entity's Board of Directors or advisory committees. Subklewe:Janssen: Consultancy, Speakers Bureau; Seattle Genetics: Research Funding; Pfizer: Consultancy; Takeda: Other: Travel support; Morphosys: Research Funding; Novartis: Consultancy, Speakers Bureau; Miltenyi Biotech: Research Funding; Bristol-Myers Squibb: Research Funding; Roche: Consultancy, Research Funding; Seagen: Research Funding; Gilead: Consultancy, Research Funding, Speakers Bureau; Celgene/BMS: Consultancy, Speakers Bureau; Amgen: Consultancy, Research Funding, Speakers Bureau. Abedi:Celgene: Consultancy, Speakers Bureau; CytoDyn: Current equity holder in publicly-traded company; Bristol Myers Squibb: Speakers Bureau; AbbVie: Speakers Bureau; Kite, a Gilead Company: Speakers Bureau; Orca Bio: Research Funding. Minnema:Bristol Myers Squibb: Speakers Bureau; Medscape: Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Wierda:AstraZeneca/Acerta Pharma. Inc.: Research Funding; AbbVie: Research Funding; Karyopharm: Research Funding; Kite, a Gilead Company: Research Funding; Bristol Meyers Squibb (Juno and Celgene): Research Funding; Sanofi: Consultancy; Genzyme: Consultancy; Cyclacel: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; Miragen: Research Funding; Sunesis: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Pharmacyclics LLC: Research Funding; Juno: Research Funding; Xencor: Research Funding; Janssen: Research Funding; GSK/Novartis: Research Funding; Gilead Sciences: Research Funding; Genentech: Research Funding. DeAngelo:Amgen: Consultancy; Autolos: Consultancy; Blueprint Medicines Corporation: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy; Shire: Consultancy; Agios: Consultancy; Servier: Consultancy; Glycomimetics: Research Funding; Gilead: Consultancy; AbbVie: Research Funding; Takeda: Consultancy; Incyte: Consultancy; Jazz: Consultancy; Forty-Seven: Consultancy; Kite, a Gilead Company: Consultancy. Stiff:Pfizer: Research Funding; Karyopharm: Research Funding; Gilead: Research Funding; Incyte: Research Funding; Cellectar: Research Funding; Kite, a Gilead Company: Research Funding; Seagen: Research Funding; Gamida Cell: Research Funding; Amgen: Research Funding; CRISPR Therapeutics: Consultancy; MorphoSys: Consultancy; Janssen: Research Funding; Bristol Myers Squibb: Research Funding; Macrogenics: Research Funding. Jeyakumar:Pfizer: Research Funding; Jazz Pharmaceuticals: Research Funding. Dong:Kite, a Gilead Company: Current Employment; Gilead Sciences: Current equity holder in publicly-traded company; GliaCure: Consultancy; Haydon et al.: Other: patents, royalties, or other intellectual property from: Patent US8598141 (Dec 03, 2013).. Adhikary:Kite, a Gilead Company: Current Employment. Zhou:Kite, a Gilead Company: Current Employment; AbbVie: Current equity holder in publicly-traded company, Other: Travel support. Schuberth:Gilead Sciences: Current equity holder in publicly-traded company, Other: Travel support; Kite, a Gilead Company: Current Employment, Other: Travel support. Faghmous:Kite, a Gilead Company: Current Employment; Gilead Sciences: Current equity holder in publicly-traded company; Roche: Current equity holder in publicly-traded company. Masouleh:Kite, a Gilead Company: Current Employment, Current equity holder in publicly-traded company, Other: Travel support; Lava: Current equity holder in publicly-traded company. Houot:Gilead: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria; MSD: Honoraria; Bristol Myers Squibb: Honoraria; Roche: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Janssen: Honoraria; ADC therapeutics: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal